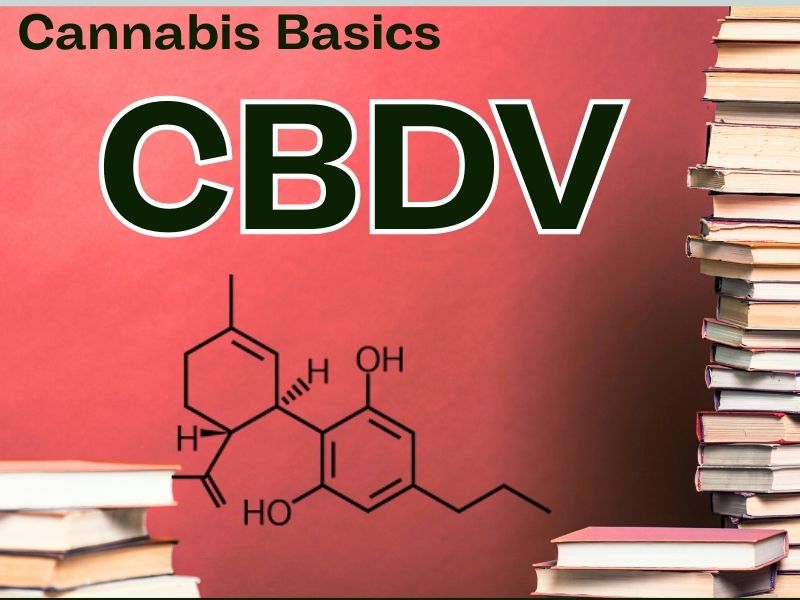
Cannabidivarin (CBDV) is an intriguing minor cannabinoid closely related to cannabidiol (CBD). While CBD has garnered significant attention for its diverse therapeutic applications, CBDV is emerging as a promising compound in its own right, especially in the field of neurological conditions. Like many minor cannabinoids, CBDV occurs naturally in relatively small quantities, typically found in specific cannabis strains originating from regions such as Asia and Africa. Strains rich in CBDV often come from landrace plants known for their unique cannabinoid profiles.
CBDV first caught scientific attention due to its structural similarity to CBD and its non-intoxicating nature. Researchers quickly became interested in its potential effects on neurological disorders, such as epilepsy, autism spectrum disorder (ASD), and neurodegenerative diseases like Huntington’s disease.
One of the most promising areas of CBDV research revolves around its anticonvulsant properties. Animal studies conducted by GW Pharmaceuticals (now part of Jazz Pharmaceuticals) have indicated that CBDV can significantly reduce seizure activity. A notable 2013 study published in the British Journal of Pharmacology showed that CBDV effectively suppressed seizures across several animal models without causing sedation or tolerance, marking it as a potentially safe treatment for epilepsy.
In addition to its anticonvulsant activity, CBDV has shown promise for autism spectrum disorders. A study published in Translational Psychiatry in 2019 demonstrated that CBDV could improve social interaction deficits and repetitive behaviors in animal models of autism. These findings suggest that CBDV may offer a targeted, low-side-effect alternative or adjunct to conventional treatments, improving the quality of life for individuals on the autism spectrum.
Moreover, the therapeutic potential of CBDV extends beyond epilepsy and autism. Its anti-inflammatory and neuroprotective properties make it particularly attractive for treating neurodegenerative diseases. Early laboratory studies indicate that CBDV may help protect brain cells by reducing inflammation and oxidative stress, which are critical factors in diseases like Alzheimer’s, Parkinson’s, and especially Huntington’s disease.

Huntington’s disease, a genetic disorder characterized by the progressive degeneration of nerve cells in the brain, currently has no cure and few effective treatments. One promising avenue for CBDV is its ability to influence neurogenesis—the formation of new neurons. Preclinical studies suggest CBDV could promote neuronal survival and improve neuroplasticity, potentially slowing the progression of Huntington’s disease or alleviating some of its devastating symptoms.
Research published in the Journal of Neurochemistry in 2018 highlighted CBDV’s potential to reduce neuroinflammation and enhance cell viability in models of Huntington’s disease. CBDV appeared to mitigate harmful inflammatory pathways and support the health and longevity of neurons, presenting a hopeful therapeutic strategy to manage or potentially slow this debilitating disease.
Furthermore, the mechanisms underlying CBDV’s beneficial neurological effects appear to involve the modulation of various neurotransmitter systems. CBDV interacts with transient receptor potential (TRP) channels, particularly TRPV1, TRPV2, and TRPA1, which are implicated in regulating neuronal excitability, inflammation, and pain perception. By influencing these channels, CBDV could stabilize neural function and offer significant therapeutic benefits in multiple neurological conditions.
Beyond its direct neurological effects, CBDV has demonstrated considerable promise for gastrointestinal health, particularly in disorders involving inflammation and discomfort. Laboratory and animal research have revealed that CBDV can significantly reduce intestinal inflammation and hypermotility, offering potential relief for conditions such as irritable bowel syndrome (IBS) and inflammatory bowel diseases (IBD), including Crohn’s disease.
Clinical observations in medical cannabis practice reinforce these preclinical findings, with patients reporting notable improvements in gastrointestinal symptoms following CBDV use. These anecdotal experiences support the need for clinical trials to confirm CBDV’s efficacy and to establish dosing and administration guidelines for optimal patient outcomes.
Interestingly, CBDV’s therapeutic profile seems to extend even further. Preliminary studies suggest it could be useful in treating nausea, pain, and mood disorders. Its broad pharmacological activities, coupled with a favorable safety profile similar to CBD, make it a versatile candidate for integrative therapies across multiple health concerns.

Given CBDV’s non-intoxicating nature, it holds significant advantages over THC-containing treatments, particularly for patients sensitive to psychoactive effects. Its safety profile, demonstrated in early studies, suggests minimal side effects, enhancing its suitability for long-term use in chronic conditions.
Despite these promising findings, clinical trials involving CBDV are still limited. Expanding research into human clinical settings remains crucial to validate its therapeutic potential fully. Notably, pharmaceutical companies such as GW Pharmaceuticals (Jazz Pharmaceuticals) have recognized CBDV’s potential, investing in research programs designed to explore its efficacy further in epilepsy and autism spectrum disorders, signaling confidence in its therapeutic future.
In conclusion, CBDV emerges as an exciting cannabinoid with substantial therapeutic potential in neurology, particularly for epilepsy, autism, and neurodegenerative diseases like Huntington’s disease. Its additional anti-inflammatory and gastrointestinal benefits further expand its potential uses. Continued research and clinical trials will undoubtedly deepen our understanding of CBDV and its role in medicine, potentially offering new hope for patients with challenging neurological conditions.